https://truthcomestolight.com/5-year-old-died-4-days-after-pfizer-shot-cdc-vaers-data-show/
VAERS data released today by the Centers for Disease Control and Prevention included a total of 927,740 reports of adverse events from all age groups following COVID vaccines, including 19,532 deaths and 146,720 serious injuries between Dec. 14, 2020, and Nov. 26, 2021.
by Megan Redshaw, The Defender
December 3, 2021
The Centers for Disease Control and Prevention today released new data showing a total of 927,740 reports of adverse events following COVID vaccines were submitted between Dec. 14, 2020, and Nov. 26, 2021, to the Vaccine Adverse Event Reporting System (VAERS). VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S
The data included a total of 19,532 reports of deaths — an increase of 283 over the previous week — and 146,720 reports of serious injuries, including deaths, during the same time period — up 3,325 compared with the previous week.
Excluding “foreign reports” to VAERS, 672,373 adverse events, including 8,986 deaths and 57,143 serious injuries, were reported in the U.S between Dec. 14, 2020, and Nov. 26, 2021.
Foreign reports are reports received by U.S manufacturers from their foreign subsidiaries. Under U.S Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 8,986 U.S. deaths reported as of Nov. 26, 20% occurred within 24 hours of vaccination, 26% occurred within 48 hours of vaccination and 61% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 454 million COVID vaccine doses had been administered as of Nov. 24. This includes 264 million doses of Pfizer, 173 million doses of Moderna and 16 million doses of Johnson & Johnson (J&J).
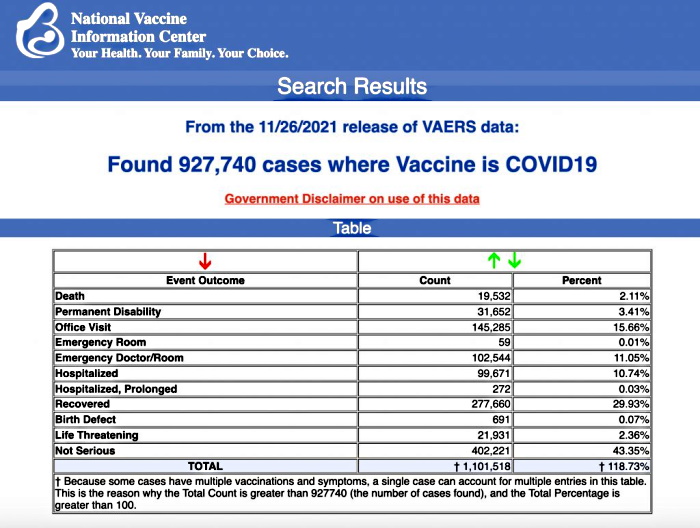
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed. Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S VAERS data from Dec. 14, 2020, to Nov. 26, 2021 for 5- to 11-year-olds show:
The second death (VAERS I.D. 1890705) occurred in a 5-year-old girl who died four days after receiving her first dose of Pfizer.
- 1,581 adverse events have been reported in the 5 to 11 age group since Nov. 1.
U.S VAERS data from Dec. 14, 2020, to Nov. 26, 2021 for 12- to 17-year-olds show:
The most recent death involves a 16-year-old girl from Georgia (VAERS I.D. 1865389) who died reportedly from a heart condition and multi-organ failure two days after receiving Pfizer’s COVID vaccine.
- 60 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 96% of cases
attributed to Pfizer’s vaccine. - 563 reports of myocarditis and pericarditis (heart inflammation) with 553 cases attributed to Pfizer’s vaccine.
- 139 reports of blood clotting disorders, with all cases attributed to Pfizer.
U.S VAERS data from Dec. 14, 2020, to Nov. 26, 2021, for all age groups combined, show:
- 19% of deaths were related to cardiac disorders.
- 54% of those who died were male, 42% were female and the remaining death reports did not include gender of the deceased.
- The average age of death was 72.7
- As of Nov. 26, 4,480 pregnant women reported adverse events related to COVID vaccines, including 1,411 reports of miscarriage or premature birth.
- Of the 3,219 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 41% to Moderna and 8% to J&J.
- 764 reports of Guillain-Barré syndrome (GBS), with 42% of cases attributed to Pfizer, 29% to Moderna and 27% to J&J.
- 2,163 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 11,334 reports of blood clotting disorders. Of those, 5,024 reports were attributed to Pfizer, 4,037 reports to Moderna and 2,222 reports to J&J.
- 3,257 cases of myocarditis and pericarditis with 2,025 cases attributed to Pfizer, 1,085 cases to Moderna and 137 cases to J&J’s COVID vaccine.
Athletes experience devastating injuries following COVID vaccines
As The Defender reported Dec. 2, several high-performing professional athletes are facing the end of their careers after COVID vaccines destroyed their health.
Florian Dagoury, a world record-holder in static breath-hold freediving, who once held his breath for a shocking 10 minutes and 30 seconds, was diagnosed with myocarditis, pericarditis and trivial mitral regurgitation after receiving Pfizer’s COVID vaccine.
Dagoury said he now struggles to reach an 8-minute breath-hold, feels an urge to breathe doing 40-minute dives, can’t keep his heart rate low and experienced a 30% decrease in his diving performance.
Veteran triathlete Antoine Méchin, 32, is also facing the potential end to his career after experiencing a pulmonary embolism after receiving Moderna’s COVID vaccine.
The symptoms, which included breathing problems and arm pain, started after the first dose, but doctors brushed off his shortness of breath as related to stress and fatigue.
Jeremy Chardy, a 34-year old professional tennis player ranked 73rd in the world, suspended his season due to a severe adverse reaction to a COVID vaccine, which left him unable to engage in intense activity.
Kyle Warner, a 29-year-old professional mountain bike racer, developed pericarditis, postural orthostatic tachycardia syndrome (POTS) and reactive arthritis following his second dose of Pfizer’s COVID vaccine.
Warner’s reaction was so severe that, as of October, he was still spending days in bed, overwhelmed by too much mental or physical exertion.
Two professional soccer players collapse during games
A professional soccer player collapsed suddenly on Nov. 25, during a Real Madrid’s Champions League game with Sheriff Tiraspol, a Moldovan soccer club, ZeroHedge reported.
Adama Traore, 26, a winger for Sherriff Tiraspol, was seen clutching his chest as he slumped to the ground in the middle of the game as medics rushed to revive him. The reasons behind Traore’s collapse and why he was suffering from chest pains have not been confirmed.
Traore’s collapse occurred the night after another player, Sheffield United’s John Fleck, went down during a match against Reading. Fleck was taken off on a stretcher after receiving lengthy treatment.
When a radio pundit questioned whether Fleck had received the COVID vaccine, his live feed to the show was cut.
A major German newspaper, Berliner Zeitung, recently published a report attempting to answer why an “unusually large number of professional and amateur soccer players have collapsed recently.”
The article listed many recent cases of players who experienced heart problems or collapsed on the field — in some cases resulting in death.
Pfizer seeks authorization for boosters shots for 16- and 17-year-olds
Pfizer CEO Albert Bourla said in a tweet on Tuesday the pharma giant, along with BioNTech, formally asked the FDA to authorize COVID booster doses for 16- and 17-year olds.
If approved, the shot would be the first booster available to people under 18.
The FDA could approve Pfizer’s booster doses for 16- and 17-year olds as soon as next week, according to people familiar with the matter.
COVID vaccines may be associated with heightened risk of myopericarditis among men
To help determine whether a correlation exists between COVID vaccines and myopericarditis, researchers tracked data from more than 268,000 adults in Massachusetts who received at least one dose of a COVID vaccine between August 2020 and May 2021.
The researchers compared the data to a control group made up of 235,000 of the same patients — from 2018 and 2019, well before they had received any doses of a COVID vaccine.
In a study published in the American Journal of Cardiology, the researchers found the age-adjusted incidence rate of myopericarditis in men was higher in the vaccinated than the control population, while the incidence rate of myopericarditis in women was the same between the vaccinated and control populations.
They also found an increased incidence of myocardial injury in both men and women in 2021 compared to 2019, although they suggested some of the apparent increase in the diagnosis of myopericarditis after vaccination may be attributable to factors unrelated to the COVID vaccines.
Moderna CEO says Omicron COVID booster could be ready by March
Moderna President Stephen Hoge said Wednesday boosters of its COVID vaccine targeting the Omicron variant could be ready for U.S authorization as early as March.
Moderna is also developing a multivalent vaccine targeting Omicron and three other COVID variants, although the shot will not be available for several more months, Forbes reported.
March is the earliest date an Omicron booster could be approved under current FDA guidelines, though the company can start manufacturing the vaccine during testing.
Hoge said he thinks existing vaccines “will be able to slow down, if not completely stop, the Omicron variant.”
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.
©December 2021 Children’s Health Defense, Inc. This work is reproduced and distributed with the permission of Children’s Health Defense, Inc. Want to learn more from Children’s Health Defense? Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. Your donation will help to support us in our efforts.
Connect with Children’s Health Defense
cover image credit: KitzD66 / pixabay

The Children’s Health Defense Team is devoted to the health of people and our planet. Their mission is to work aggressively to reduce exposure to all sources of mercury, hold those accountable who failed to protect our planet and people from unnecessary exposure, restore health to those who have been harmed, and make sure this tragedy never happens again.
They do what they do for many reasons. Some have children who have been injured. Some have seen an injustice and feel the need to step in where others fear to tread. Some are focused on the demand for scientific integrity, others on improving the health of this, and future, generations.
See: Why We Do What We Do
Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. CHD is planning many strategies, including legal, in an effort to defend the health of our children and obtain justice for those already injured. Your support is essential to CHD’s successful mission.
The Defender is Children’s Health Defense’s news and views website. It features content aligned with the organization’s mission to end childhood health epidemics. Sign up for updates.
Children’s Health Defense Europe is the Belgium-based Europe chapter of CHD.


